Mixtures of concentrated nitric and sulfuric acids are used in the preparation of explosives and propellants for military and civilian use. Tight control of the amount of the individual acids is necessary in order to obtain products of consistent composition and performance. Rapid feedback to the production department of analyses is essential to permit corrective action to out of specification nitrating mixtures without losing valuable time. Traditional manual analytical procedures may not be capable of delivering results within a timely manner, and so automated analytical procedures may be considered. Further, traditional manual methods may be subject to analyst error, and a high degree of training and supervision may be required in order to obtain results of sufficient accuracy and precision.
A new automated TET analytical protocol for the analysis of sulfuric, nitric and nitrous acids has been developed, employing the Metrohm 859 Titrotherm thermometric titration system. Nitrous acid is a by-product of the nitration process, and its presence has to be compensated, in order to obtain an accurate value for the nitric acid content. The protocol requires two separate titration sequences. In the first titration, the HNO2 content is determined by a direct TET, employing 0.1 mol/L KMnO4 as titrant. The result is saved by the software. In the second sequence, the sulfuric acid content of the sample is determined by TET with 1 mol/L BaCl2, and the result saved. The second titration of the sequence then starts. This is the determination of the Total Acid content, by TET with 2 mol/L NaOH. In the calculation at the end of the second titration, the HNO3 content is determined by subtracting the H2SO4 and HNO2 contents, after converting the results to an HNO3 equivalent.
A complete H2SO4-HNO3-HNO2 analysis can be completed in less than 7 minutes. For higher analytical productivity, a sample changer can be used. The analyst only needs to weigh the samples into the titration vessels, and place them in the rack of the sample changer. All other steps are fully automated. The procedure is also suitable for completely automated on-line analysis.
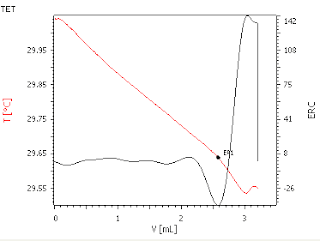
Typical TET titration plots are illustrated in Figs. 1, 2 and 3. The solution temperature as a function of titrant delivered is shown as a red curve. The endpoint is accurately located from the inflection in the second derivative curve.
Fig. 2. TET plot - titration of Total Acids (H2SO4+HNO3+HNO2) with 2 mol/L NaOH
It should be emphasized that these three different titrations were all accomplished with the same sensor. This is simply a highly sensitive, rapidly responding electronic thermometer. It requires no calibration and minimal maintenance, and is thus ideally suited for routine process and quality control applications.
A new automated TET analytical protocol for the analysis of sulfuric, nitric and nitrous acids has been developed, employing the Metrohm 859 Titrotherm thermometric titration system. Nitrous acid is a by-product of the nitration process, and its presence has to be compensated, in order to obtain an accurate value for the nitric acid content. The protocol requires two separate titration sequences. In the first titration, the HNO2 content is determined by a direct TET, employing 0.1 mol/L KMnO4 as titrant. The result is saved by the software. In the second sequence, the sulfuric acid content of the sample is determined by TET with 1 mol/L BaCl2, and the result saved. The second titration of the sequence then starts. This is the determination of the Total Acid content, by TET with 2 mol/L NaOH. In the calculation at the end of the second titration, the HNO3 content is determined by subtracting the H2SO4 and HNO2 contents, after converting the results to an HNO3 equivalent.
A complete H2SO4-HNO3-HNO2 analysis can be completed in less than 7 minutes. For higher analytical productivity, a sample changer can be used. The analyst only needs to weigh the samples into the titration vessels, and place them in the rack of the sample changer. All other steps are fully automated. The procedure is also suitable for completely automated on-line analysis.
Typical TET titration plots are illustrated in Figs. 1, 2 and 3. The solution temperature as a function of titrant delivered is shown as a red curve. The endpoint is accurately located from the inflection in the second derivative curve.
Fig.1. TET plot - titration of H2SO4 in nitrating mixture with 1 mol/L BaCl2
Fig. 2. TET plot - titration of Total Acids (H2SO4+HNO3+HNO2) with 2 mol/L NaOH
Fig. 3. TET plot - titration of HNO2 with 0.1 mol/L KMnO4