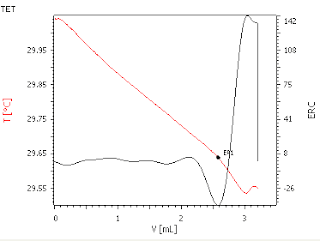
Good agreement with result obtained by conventional titrimetric method
Many crude oils contain a range of acidic substances which can cause corrosion damage to refineries during processing. These acidic substances can be categorized as “napthenic acids” which have been defined as an “unspecific mixture of several cyclopentyl and cyclohexyl carboxylic acids with molecular weight of 120 to well over 700 atomic mass units”. Sulfur-containing acids may also be present, and can contribute to corrosion problems. The bulk acid content of an oil is defined by its AN (Acid Number) value, defined as mg KOH/g of oil. The unit price of a crude oil can be discounted significantly according to its AN, so it is important to be able to analyze the AN value with accuracy and precision.
To date, the conventional method to determine the AN value of crude oils has been a potentiometric titration based on ASTM method D664. The success of this method is dependent on the type of crude oil analyzed, and the skill of the analyst in identifying problems which may affect the result. Further, the performance of the pH probe is affected by dehydration and fouling of the sensor glass membrane, as well as its reference junction. Potentiometric pH probes may require cleaning and regeneration (rehydration) of the sensor after only a few titrations. In contrast to the potentiometric method, the thermometric probe requires no conditioning and minimal maintenance. It may be stored dry between titrations, and is always ready for service. It is thus very suitable for use in remote locations with minimal laboratory facilities.
In thermometric endpoint titrations (TET), the endpoint
is determined by the rate of change of temperature of the titration solution as
the titrant is added. In the case of non-aqueous titrations of weakly acidic
species, the temperature inflection at the endpoint is too low to be detected
reliably. In this case, a thermometric indicator (paraformaldehyde) is
employed. At the endpoint, the first trace of excess KOH titrant catalyzes the
endothermic decomposition of the paraformaldehyde. This provides a reliable
marker for the endpoint. The titration is carried out in an anhydrous mixture of xylene and 2-propanol. There is no need to add water to the solvent, as is the case with potentiometric titrations.
A sample of a very dark, highly viscous crude oil
African origin (Doba, Chad) was provided for evaluation of the procedure.
Reagents:
Reagents:
2. Solvent: 1:1 mixture of xylene with
2-propanol
3. Thermometric indicator: paraformaldehyde
(e.g., Sigma Aldrich cat. no. 158127)
4. Standard: c(C7H6O2)
= 0.1 mol/L benzoic acid in 2-propanol
Method Outline:
Method Outline:
Approximately 3 g of crude oil is weighed into a
titration vessel, and 30 mL of solvent mixture added. Approximately 0.5 g of
paraformaldehyde is then added. The titration is then commenced, and stopped
after appearance of the endpoint inflection.
In the case of the semi-automated titrations reported on here, the paraformaldehyde is added as a powder. A level 1/8th
kitchen teaspoon measure was used to dispense the paraformaldehyde. In the case
of fully automated titrations employing a sample changer, the paraformaldehyde
is slurried with the solvent mixture, and delivered by peristaltic pump as part
of the titration program. The titration can be stopped automatically after the
endpoint appears.
Results:
Results:
Due to the small amount of oil provided, the average AN
was computed from a limited number of determinations, with sample masses ranging from approximately 0.5 to 4 g.. Individual
results were 4.1, 4.3, 4.2, 4.2, 4.2 and 4.3 with an average of 4.2±0.09 mg KOH/g. The result obtained appears to be
insensitive to the amount of sample mass, at least within the range tested.
The customer recorded a value of 4.4 mg/100g KOH by a procedure similar to that specified in ASTM D664.
The customer recorded a value of 4.4 mg/100g KOH by a procedure similar to that specified in ASTM D664.
TET plot of acids in crude oil in 1:1 xylene/2-propanol, using c(KOH) = 0.1 mol/L as titrant.