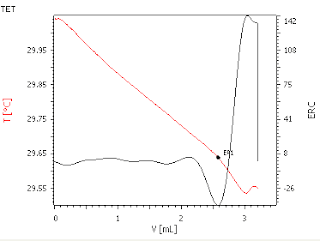
At long last, ASTM D8045 is here!
Way back in 2002, some Brazilian chemists came up with a smart new method to measure the free fatty acid (FFA) content of vegetable oils using thermometric endpoint titrimetry (TET) (1). I tried it out, and it worked like a charm. It was fast, accurate, and very precise, all the things you need in process and quality control. The endpoints are highly reliable, and best of all, the sensor doesn't crud up like a potentiometric pH electrode, and require cleaning and rehydration every other titration or so. The probe can be stored dry, and is ready for use 24/7. I thought, this method is essentially about the determination of weakly acidic species in a non-aqueous environment. I had in mind a replacement for ASTM D664, the potentiometric titration procedure for Acid Number in lubricating and other mineral oils. For those of you who have had the misfortune to work with this purely dreadful procedure, you'll know what I mean. Let's face it, glass membrane pH probes just aren't meant to operate in a non-aqueous environment. The glass membrane has to be kept in a hydrated state, and the reference junction has to be kept clean. Operating in a dehydrating, oily medium isn't really their thing. Yes, I know that companies have spent years and a lot of money developing pH probes which do work a bit better in non-aqueous solutions, but really, they're a compromise at best. All hail to those clever Brazilians for coming up with a procedure which is really useful!
It was 2005 when I adapted and tried out the new TET method on both vegetable and mineral oils. When our company Multitrator sold the TET enabling technology to Metrohm, it became part of the technology package we handed over. Fast forward to early 2008, when I introduced the method to Metrohm USA..Fast forward again to July 2016 when ASTM finally approved the procedure for the determination of acidity in oils, with the title: ASTM D8045-16 Standard Test Method for Acid Number of Crude Oils and Petroleum Products by Catalytic Thermometric Titration. Along the way, I did a bit of tweaking around the edges (improving the solvent mixture, figuring out a way to automate the addition of the thermometric indicator/catalyst), but really, the principle is the same as that outlined in the original paper.
It would be nice to sit back and relax, but there's more work to done. Firstly this procedure needs to be adopted in industries which make and use edible fats and oils; after all, it was originally intended to analyse those materials. Secondly, there is a crying need in the analysis of lubricating oils to replace the potentiometric titration for the analysis of Base Number. The potentiometric method suffers the same sorts of problems evident in the Acid Number determination - most of which are caused by the probe. I worked up another TET method for Base Number using another thermometric indicator. The idea was taken from a paper published over 40 years ago (2). I'm hoping that this procedure can be approved by ASTM more rapidly than that for Acid Number!. It is also a procedure that has wider uses than just the analysis of lubricating oils. I have tested it out, and it is excellent for the determination of purity of weak organic bases in non-aqueous media. The titrant is exactly the same as used for the potentiometric procedure, and can be bought off the shelf.
References.
1. M. J. D. Carneiro, M. A. Feres Júnior, and O. E. S. Godinho. Determination of the acidity of oils using paraformaldehyde as a thermometric end-point indicator. J. Braz. Chem. Soc. 13 (5) 692-694 (2002)
1. M. J. D. Carneiro, M. A. Feres Júnior, and O. E. S. Godinho. Determination of the acidity of oils using paraformaldehyde as a thermometric end-point indicator. J. Braz. Chem. Soc. 13 (5) 692-694 (2002)
2. E. J. Greenhow and L. E. Spencer (1973) Ionic polymerisation as a means of endpoint
indication in non-aqueous thermometric titrimetry. Part 1. The determination of
organic bases. Analyst, 98,
81-89



.jpg)
.JPG)







